In my last post, I introduced the GEMSTONE method for single-scan selective excitation of overlapped multiplets, and I showed how GEMSTONE can be combined with the NOESY mixing scheme to create an “ultra selective” 1D NOESY experiment. As I mentioned there, GEMSTONE can also be combined with TOCSY, and that is the subject of the current post.
GEMSTONE-TOCSY pulse sequence
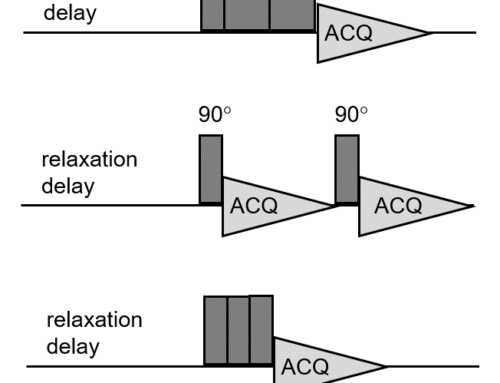
Figure 1 shows the basic GEMSTONE-TOCSY pulse sequence, which is essentially a concatenation of the basic GEMSTONE sequence with a suitable TOCSY mixing scheme (a DISPSI2 spinlock plus some additional gradients and adiabatic pulses to suppress undesirable zero-quantum coherences).

Figure 1. GEMSTONE-TOCSY pulse sequence
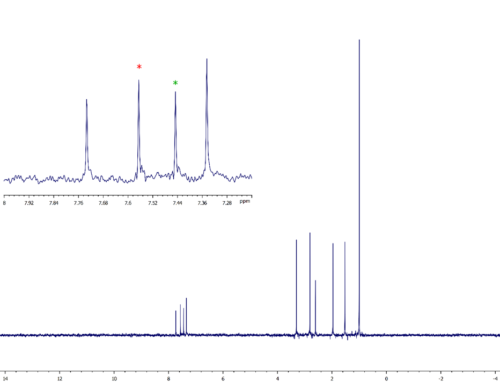
To illustrate the use of GEMSTONE-TOCSY, figure 2 shows some spectra I collected on a sample of β-estradiol. I measured separate GEMSTONE-TOCSY spectra for each of the three overlapped signals from hydrogens 16α, 12β and 7β. As you can see, GEMSTONE-TOCSY is selective enough to excite each signal individually and therefore observe independently the TOCSY correlations from each. For comparison, I also collected a standard 1D TOCSY spectrum – in this case, the standard experiment lacks the selectivity to excite only one of the signals at a time, so the spectrum shows TOCSY correlations from all three hydrogens to other hydrogens in their respective spin systems.

Figure 2. Comparison of GEMSTONE-TOCSY and conventional 1D TOCSY. The data were collected on a sample of β-estradiol using a JEOL ECZ500R spectrometer equipped with a ROYAL HFX probe and processed using JASON software. Each measurement comprised 32 scans, with an experiment time of 4.5 minutes. The signal selectively excited in each experiment is indicated in red. In the case of the standard experiment, individual excitation of 16α, 12β and 7β was not possible and all three were excited simultaneously.
To find out more about GEMSTONE experiments, or to obtain copies of the GEMSTONE pulse programs for JEOL ECZ spectrometers, contact us at the usual address!
References
- Kiraly, N. Kern, M. P. Plesniak, M. Nilsson, D. J. Procter, G. A. Morris and R. W. Adams, Angew. Chem. Int. Ed. 2021, 60, 666–669.
- Kiraly, M. Nilsson, G. A. Morris and R. W. Adams, Chem. Commun. 2021, 57, 2368-2371.